The stage 0 framework is based on three steps:
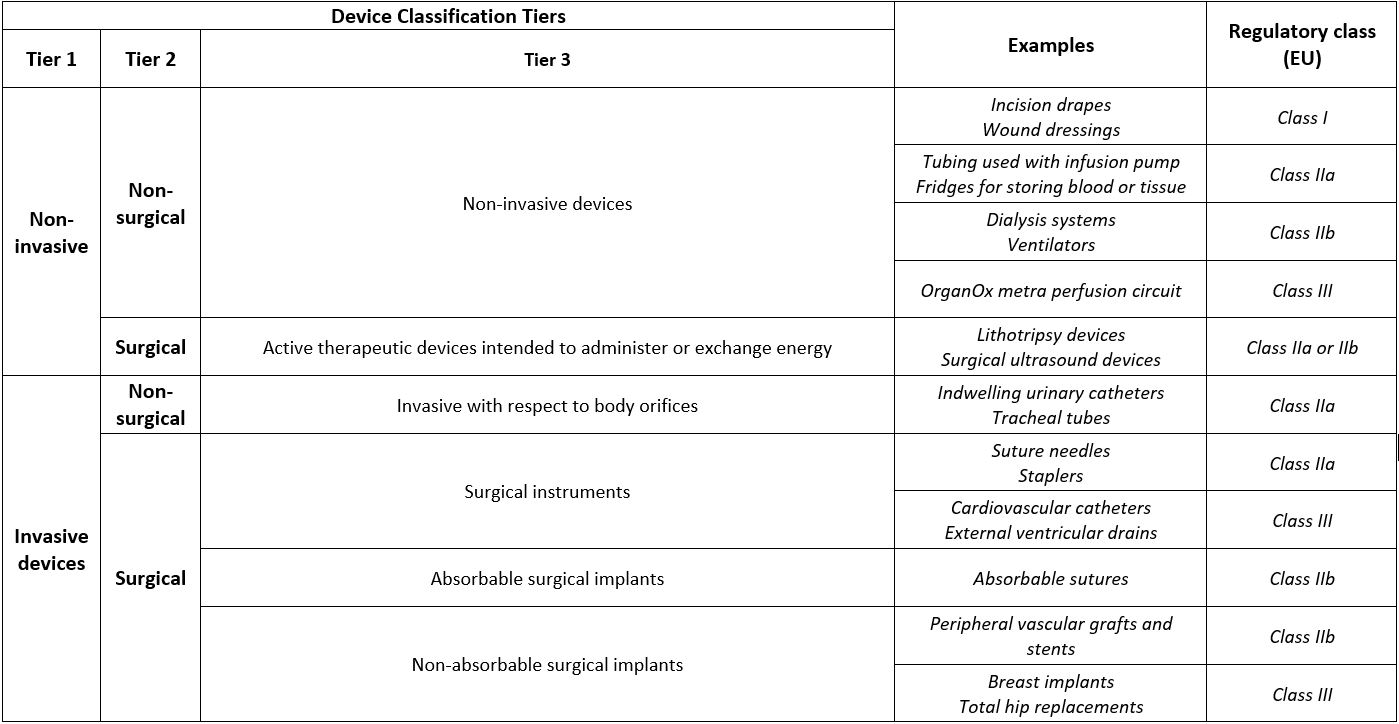
The device classification is a 3-tiered descriptive taxonomy, based on what the devices do, and therefore, provides a reflection of the potential harm a device may pose:
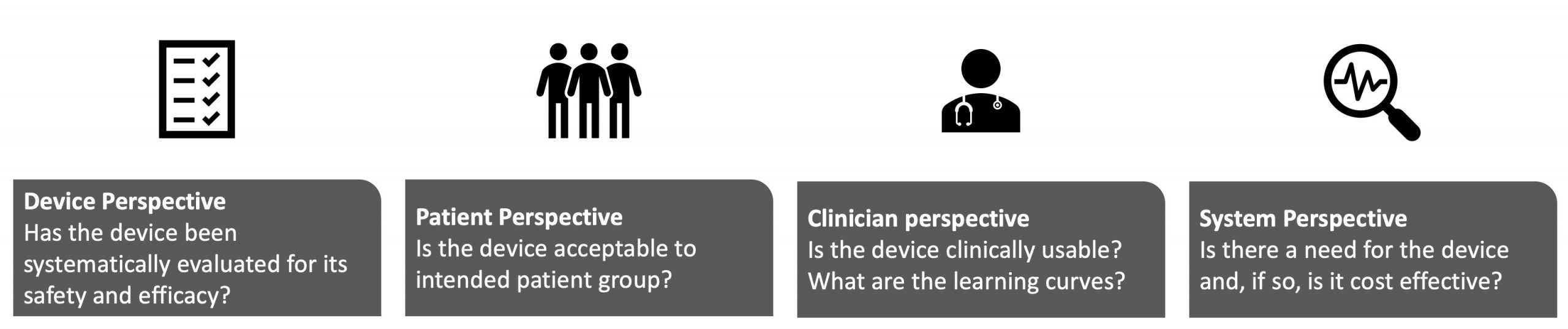
The studies that may be conducted to evaluate a device fall into 4 perspectives: device, patient, clinician, and system.
The risk a device may pose may be assessed using a Failure Modes and Effects Analysis (FMEA). For each risk, it can be categorized according to its probability and severity. The results of this FMEA analysis can be used to achieve a ‘proportionate’ evaluation that balances innovation and patient safety.
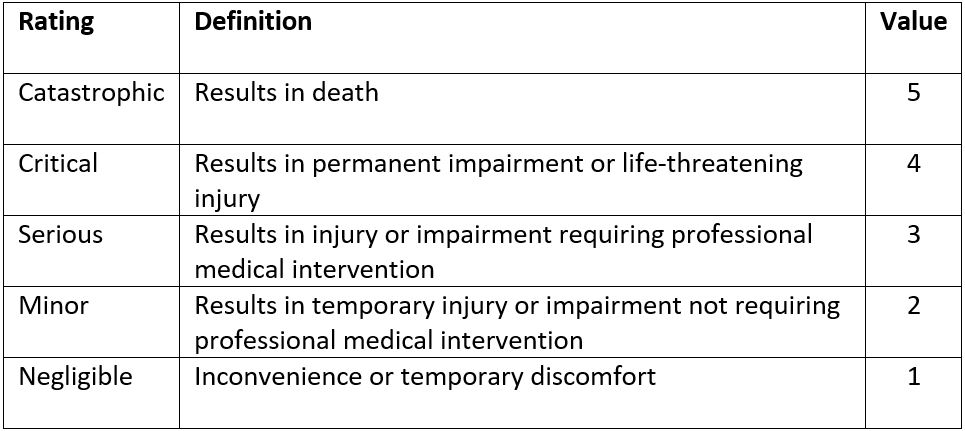
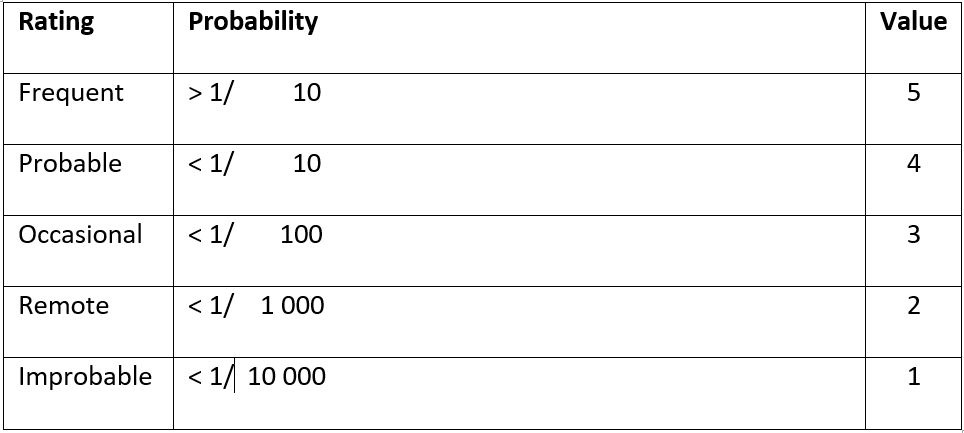
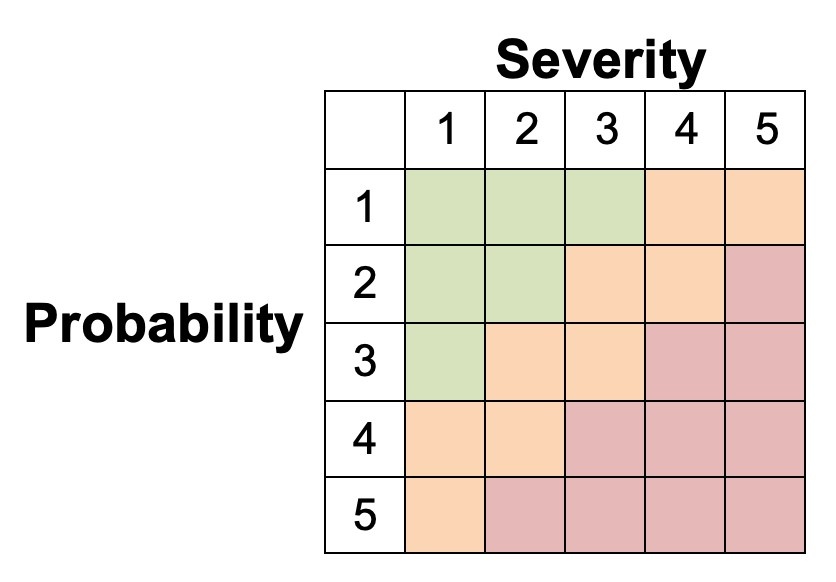
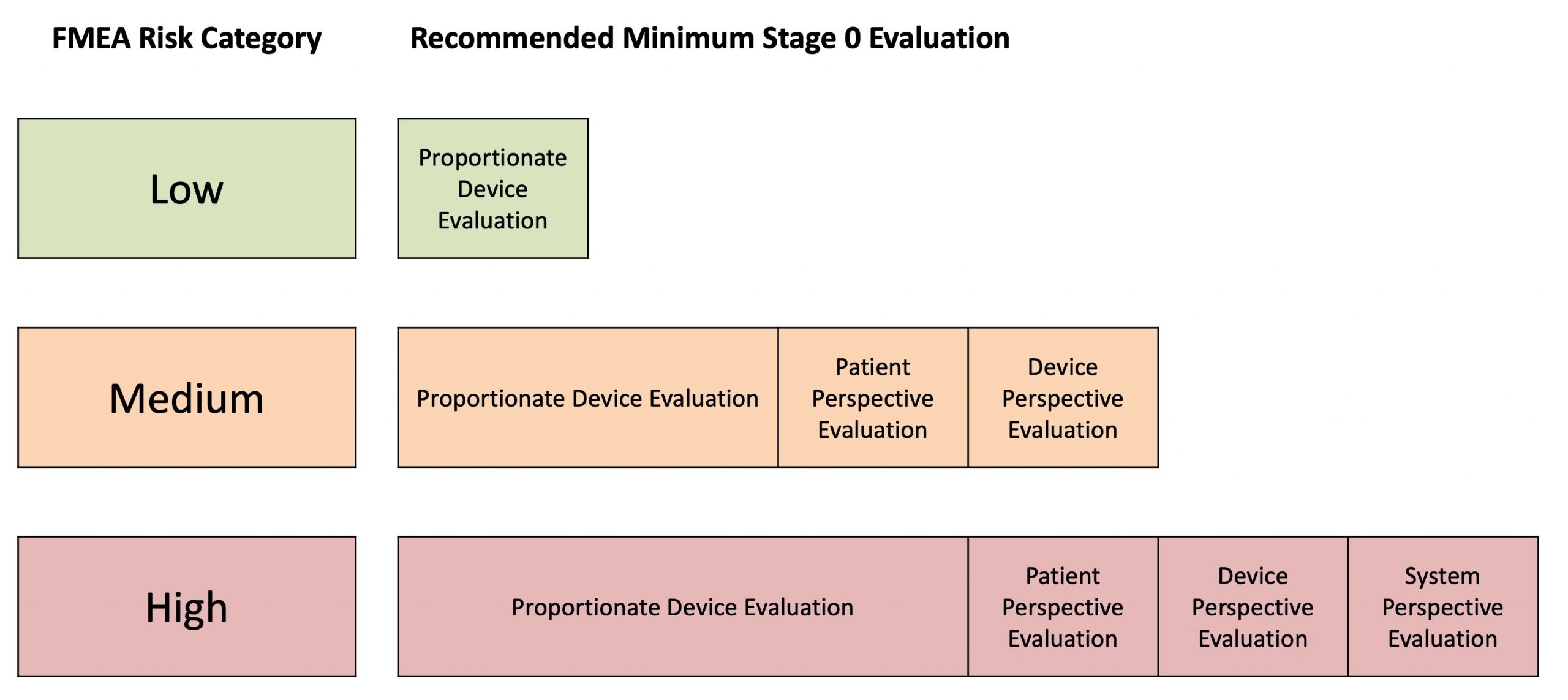
The extent of this proportionate evaluation can be guided by the figure below, which higher risk devices requiring a more thorough evaluation prior to Stage 1 studies.
Reference