IDEAL recommendations for study design and reporting in order to improve evidence on surgical and interventional therapy innovation.
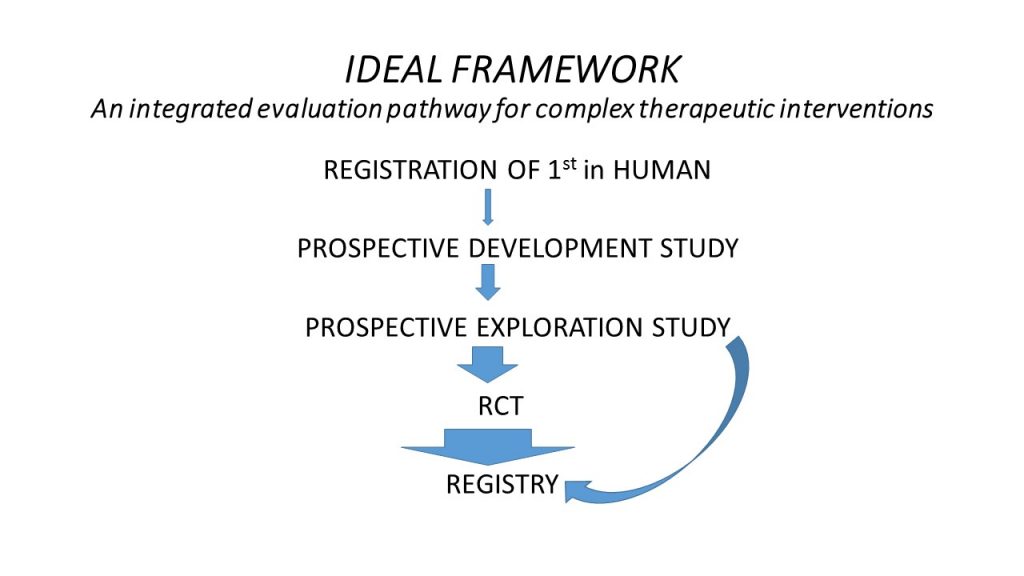 The IDEAL collaboration has endorsed a number of suggestions for specific study designs and reporting standards which are recommended at different stages in the Framework. These suggestions are underpinned by a series of general principles for design and reporting, which are based on the different questions to be addressed and the challenges faced at each stage in the process
The IDEAL collaboration has endorsed a number of suggestions for specific study designs and reporting standards which are recommended at different stages in the Framework. These suggestions are underpinned by a series of general principles for design and reporting, which are based on the different questions to be addressed and the challenges faced at each stage in the process