On the one hand, any first-in human studies carry obvious risks. On the other hand, only 10% of medical devices make it from development to stage 1. Stage 0 provides a framework for robust and transparent evaluation of devices, following the principle that the more invasive and high risk a device is, the greater proof required of their safety and effectiveness before progression to clinical studies.
The stage 0 framework is based on three steps:
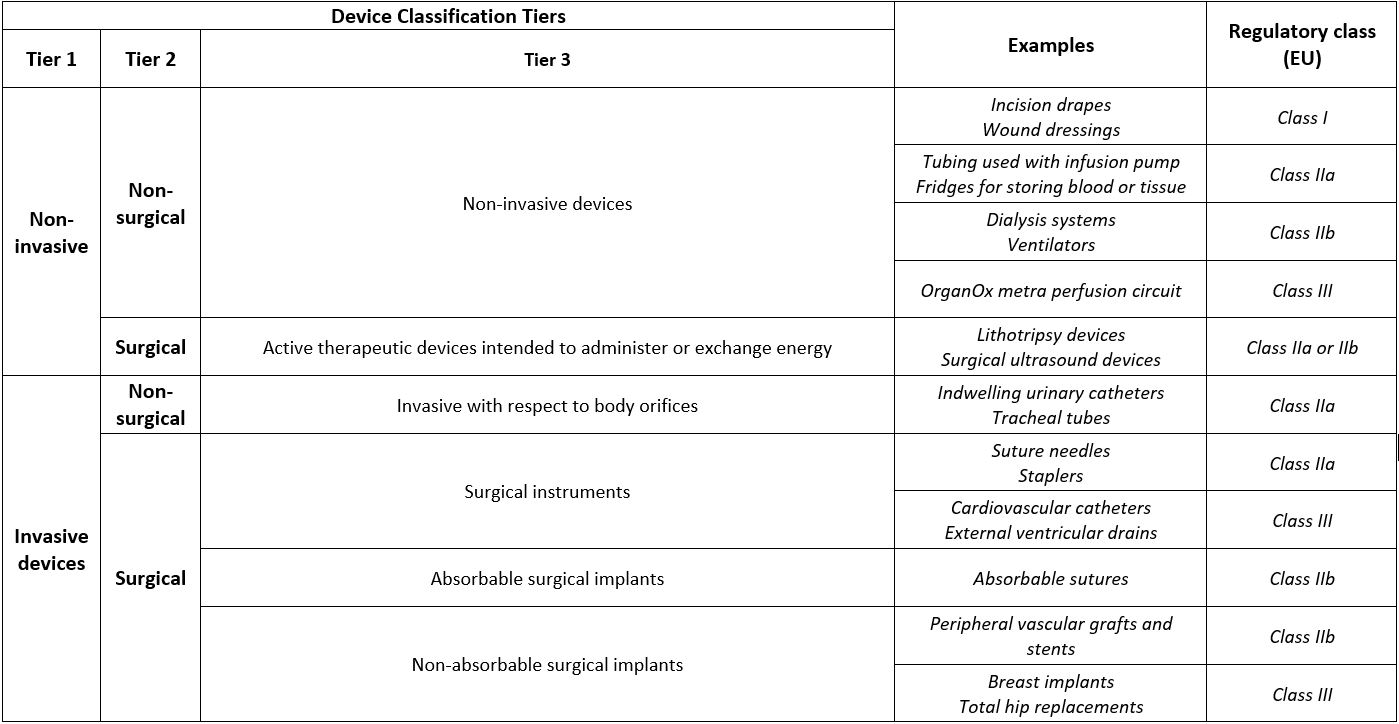
The device classification is a 3-tiered descriptive taxonomy, based on what the devices do, and therefore, provides a reflection of the potential harm a device may pose:
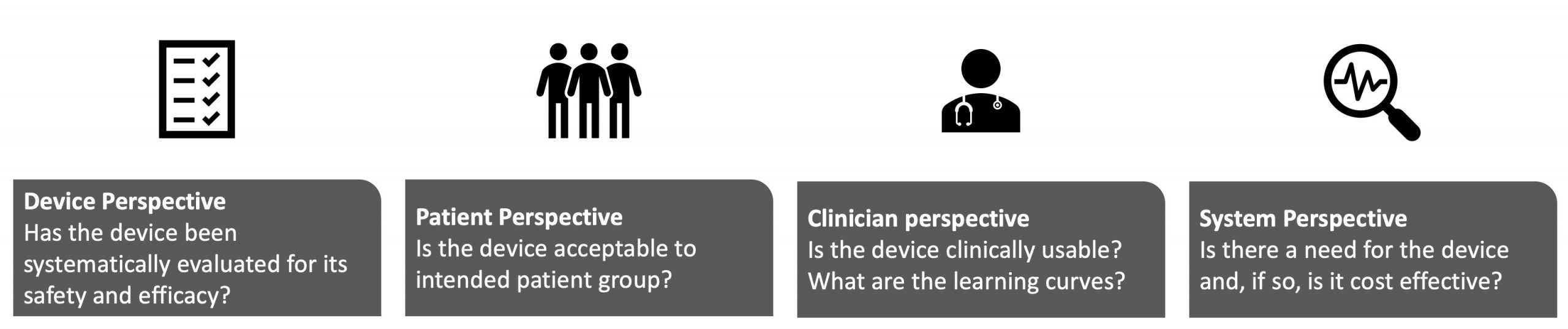
The studies that may be conducted to evaluate a device fall into 4 perspectives: device, patient, clinician, and system.
The risk a device may pose may be assessed using a Failure Modes and Effects Analysis (FMEA). For each risk, it can be categorized according to its probability and severity. The results of this FMEA analysis can be used to achieve a ‘proportionate’ evaluation that balances innovation and patient safety.
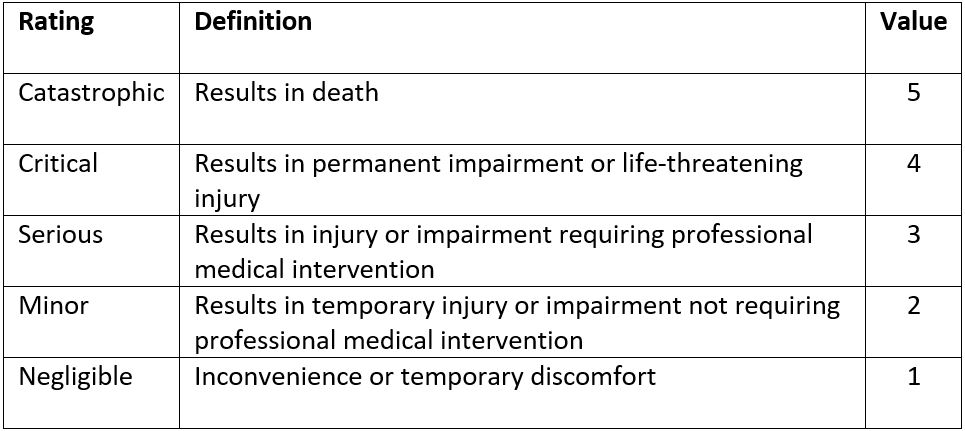
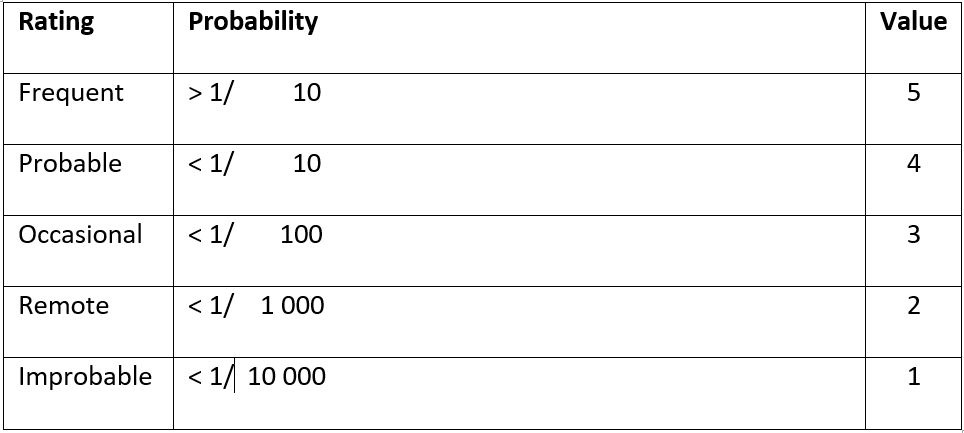
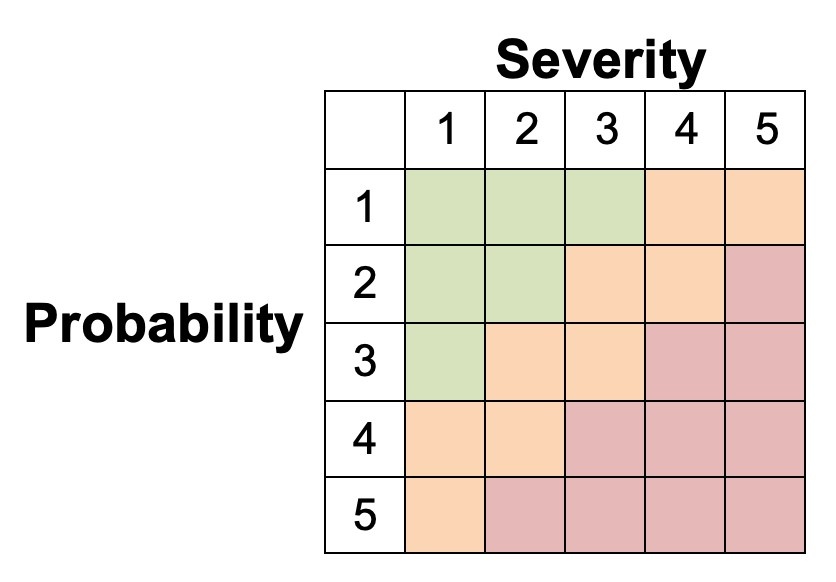
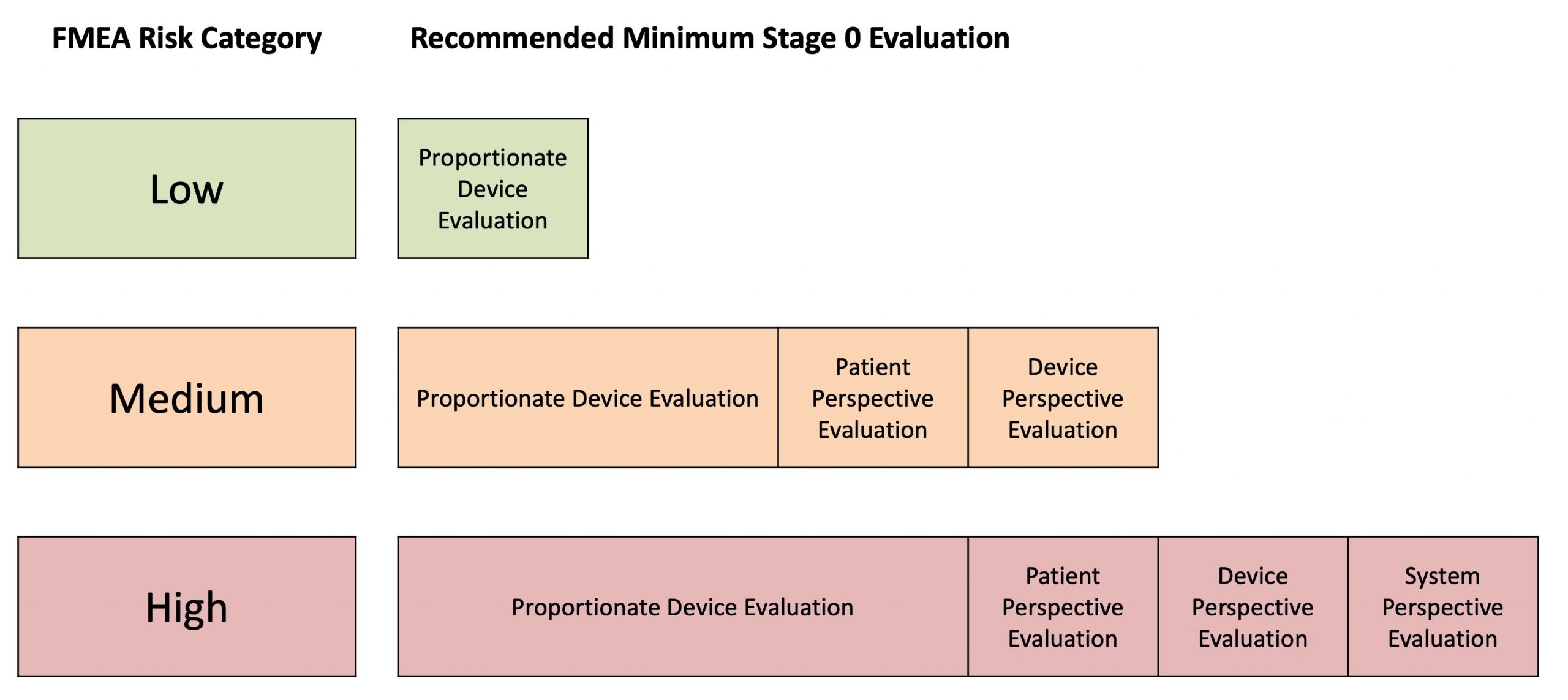
The extent of this proportionate evaluation can be guided by the figure below, which higher risk devices requiring a more thorough evaluation prior to Stage 1 studies.
Marcus, H.J., Bennett, A., Chari, A., Day, T., Hirst, A., Hughes-Hallett, A., Kolias, A., Kwasnicki, R.M., Martin, J., Rovers, M. and Squire, S.E., 2022. IDEAL-D framework for device innovation: a consensus statement on the preclinical stage. Annals of surgery, 275(1), pp.73-79.